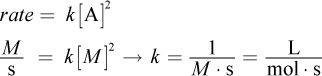MCAT Math Question 14: Answer and Explanation
Home > MCAT Test > MCAT math practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT math practice tests.
Question: 14
14. The rate of a reaction is calculated as a change in concentration per time. What are the units of the rate constant, k, in a reaction that is second order overall with respect to one species? (Note: A second-order reaction of this type has a rate law with the form rate = k[A]2, where [A] is the concentration of the species.)
- A.
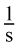
- B.
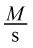
- C.
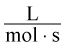
- D.
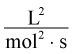
Correct Answer: C
Explanation:
According to the question stem, the rate of a reaction is measured as a change in concentration over time, and thus has the units 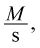
where M (molarity) is measured in moles per liter. However, the rate of the reaction is equal to a rate constant times the concentrations of certain reactants squared. In this case, we know the units of everything except the rate constant and must solve for its units: