MCAT General Chemistry Question 97: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 97
7. For a certain chemical process, 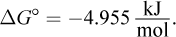 What is the equilibrium constant Keq for this reaction? (Note:
What is the equilibrium constant Keq for this reaction? (Note: 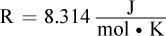 )
)
- A. Keq = 1.0
- B. Keq = 7.4
- C. Keq = 8.9
- D. Keq = 10
Correct Answer: B
Explanation:
Solve this question using the equation ΔG°rxn = -RT ln Keq. ΔGrxn° is 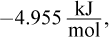
R is 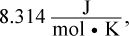
and T = 298 K because the reaction is occurring under standard conditions. Because ΔG°rxn uses kilojoules in its units and R uses joules, one will have to be converted. Plugging into the equation, we get:
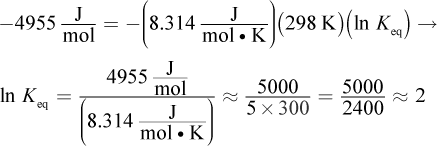
If ln Keq = 2, then Keq = e2. e is approximately 2.7, so we're looking for a number between 4 (22) and 9 (32). Both choices (B) and (C) fit these criteria; however, 8.9 is very close to 9, so we can assume that its square root is very, very close to 3. The answer choice should be a bit smaller, so choice (B), 7.4, is correct.
