MCAT General Chemistry Question 85: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 85
10. In a sealed 1 L container, 1 mole of nitrogen gas reacts with 3 moles of hydrogen gas to form 0.05 moles of NH3 at equilibrium. Which of the following is closest to the Kc of the reaction?
- A. 0.0001
- B. 0.001
- C. 0.01
- D. 0.1
Correct Answer: A
Explanation:
The first step to answering this question is to write out the balanced equation for the reaction of H2 and N2 to produce NH3: N2 + 3 H2 - 2 NH3. This means that Kc is equal to 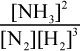 . Because the volume is 1 L, the amount of each gas (in moles) is equal to the value of the concentration of each gas (in M). If we start with 1 mole N2 and 3 moles H2, some amount x of N2 will react with 3x amount of H2 to form 2x amount of ammonia according to their stoichiometric coefficients. The amount of ammonia at equilibrium is given here as 0.05 M; thus, 2x = 0.05 and x = 0.025. Thus, the concentration of N2 at equilibrium is 1 - 0.025 = 0.975 M, and the concentrations of H2 at equilibrium is 3 - 3 × 0.025 = 2.925 M. Note that these changes are negligible because the answer choices differ by powers of ten. Thus, we can plug into the Keq expression to get
. Because the volume is 1 L, the amount of each gas (in moles) is equal to the value of the concentration of each gas (in M). If we start with 1 mole N2 and 3 moles H2, some amount x of N2 will react with 3x amount of H2 to form 2x amount of ammonia according to their stoichiometric coefficients. The amount of ammonia at equilibrium is given here as 0.05 M; thus, 2x = 0.05 and x = 0.025. Thus, the concentration of N2 at equilibrium is 1 - 0.025 = 0.975 M, and the concentrations of H2 at equilibrium is 3 - 3 × 0.025 = 2.925 M. Note that these changes are negligible because the answer choices differ by powers of ten. Thus, we can plug into the Keq expression to get 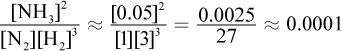 .
.
