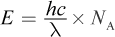MCAT General Chemistry Question 6: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 6
6. An electron returns from an excited state to its ground state, emitting a photon at λ = 500 nm. What would be the magnitude of the energy change if one mole of these photons were emitted? (Note: h = 6.626 × 10-34 J·s)
- A. 3.98 × 10-21 J
- B. 3.98 × 10-19 J
- C. 2.39 × 103 J
- D. 2.39 × 105 J
Correct Answer: D
Explanation:
While daunting at first, the problem requires the MCAT favorite equation 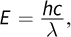 where h = 6.626 × 10-34 J·s (Planck's constant),
where h = 6.626 × 10-34 J·s (Planck's constant), 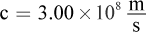 is the speed of light, and λ is the wavelength of the light. This question asks for the energy of one mole of photons, so we must multiply by Avogadro's number, NA = 6.02 × 1023 mol-1.
is the speed of light, and λ is the wavelength of the light. This question asks for the energy of one mole of photons, so we must multiply by Avogadro's number, NA = 6.02 × 1023 mol-1.
The setup is: