MCAT General Chemistry Question 227: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 227
5. The equilibrium expression below corresponds to which of the following reactions?
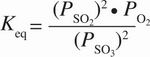
- A. 2 SO2(aq) + O2(g)
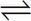 2 SO3(aq)
2 SO3(aq) - B. 2 SO3(aq)
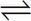 2 SO2(aq) + O2(g)
2 SO2(aq) + O2(g) - C. 2 SO2(g) + O2(g)
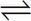 2 SO3(g)
2 SO3(g) - D. 2 SO3(g)
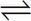 2 SO2(g) + O2(g)
2 SO2(g) + O2(g)
Correct Answer: D
Explanation:
D The expression is in terms of partial pressure, so all components must be gaseous, eliminating choices A and B. An equilibrium expression has products in the numerator and reactants in the denominator, eliminating choice C. The exponents correspond to the stoichiometric coefficients of the balanced equation, so choice D is correct.
