MCAT General Chemistry Question 14: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 14
14. Consider the two sets of quantum numbers shown in the table, which describe two different electrons in the same atom.
| n | l | ml | ms |
| 2 | 1 | 1 | 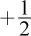 |
| 3 | 1 | -1 | 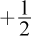 |
Which of the following terms best describes these two electrons?
- A. Parallel
- B. Opposite
- C. Antiparallel
- D. Paired
Correct Answer: A
Explanation:
The terms in the answer choices refer to the magnetic spin of the two electrons. The quantum number ms represents this property as a measure of an electron's intrinsic spin. These electrons' spins are parallel, in that their spins are aligned in the same direction (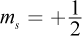 for both species).
for both species).
