MCAT General Chemistry Question 117: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 117
12. A gas at a temperature of 27°C has a volume of 60.0 mL. What temperature change is needed to increase this gas to a volume of 90.0 mL?
- A. A reduction of 150°C
- B. An increase of 150°C
- C. A reduction of 40.5°C
- D. An increase of 40.5°C
Correct Answer: B
Explanation:
We will use Charles's law. First, we must convert the temperature to kelvins by adding 273 to get 300 K as the initial temperature. Think of this as a proportionality: If the volume is multiplied by 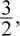 the temperature will also have to be multiplied by
the temperature will also have to be multiplied by 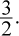 Thus the final temperature is 450 K, which represents a 150 K increase.
Thus the final temperature is 450 K, which represents a 150 K increase.
