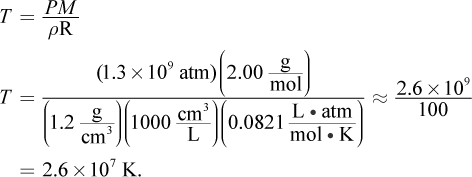MCAT General Chemistry Question 115: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 115
10. Given that the gases at the center of the sun have an average molar mass of 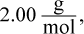 compressed to a density of
compressed to a density of 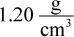 under 1.30 × 109 atm of pressure, what is the temperature at the center of the sun?
under 1.30 × 109 atm of pressure, what is the temperature at the center of the sun?
- A. 2.6 × 104 K
- B. 2.6 × 106 K
- C. 2.6 × 107 K
- D. 2.6 × 1010 K
Correct Answer: C
Explanation:
The ideal gas law can be modified to include density (ρ) because the number of moles of gas, n, is equal to the mass divided by the molar mass. Thus,
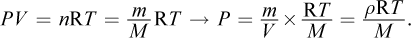
Isolating for temperature, we get: