GRE Problem Solving Question 1686
Home > GRE Test > GRE Problem Solving Questions
Next steps
- Use your browser's back button to return to your test results.
- Do more GRE Problem Solving Questions.
Source: BOOST
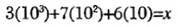
Notes:Drawn to scale. Each solution referred to consists of water and only one of the compounds.
Percent concentration means percent of compound per unit of volume of solution. Assume that the
volume of the solution is equal to the sum of the volume of the water and the volume of the compound added to the water.
A solution containing approximately what percent concentration of methanol has a freezing point of-15°C?
- A 40%
- B 30%
- C 25%
- D 18%
- E 15%
