MCAT Physics Question 40: Answer and Explanation
Home > MCAT Test > MCAT physics practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT physics practice tests.
Question: 40
10. A certain substance has a specific heat of 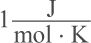 and a melting point of 350 K. If one mole of the substance is currently at a temperature of 349 K, how much energy must be added in order to melt it?
and a melting point of 350 K. If one mole of the substance is currently at a temperature of 349 K, how much energy must be added in order to melt it?
- A. More than 1 J
- B. Exactly 1 J
- C. Less than 1 J but more than 0 J
- D. Less than 0 J
Correct Answer: A
Explanation:
To find the amount of heat needed to bring the substance to its melting point, you can use the specific heat. To heat one mole of the substance one unit kelvin, it would take 1 J of heat. After the substance reaches its melting point, additional heat is needed to actually induce the phase change. Therefore, the total amount of heat required is greater than 1 J.
