MCAT Physics Question 35: Answer and Explanation
Home > MCAT Test > MCAT physics practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT physics practice tests.
Question: 35
5. How much heat is required to completely melt 500 g gold earrings, given that their initial temperature is 25°C? (The melting point of gold is 1064°C, its heat of fusion is 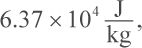 and its specific heat is
and its specific heat is 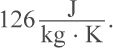
- A. 15 kJ
- B. 32 kJ
- C. 66 kJ
- D. 97 kJ
Correct Answer: D
Explanation:
First determine how much heat is required to raise the temperature of the gold earrings to the melting point of gold. Then, calculate the heat required to actually melt the earrings (the latent heat). The total heat required to melt the earrings completely will be the sum of the two heats. The heat required to raise the temperature of the earrings from 25°C to 1064°C is
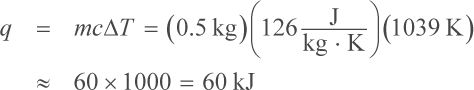
Thus, it takes about 60 kJ of heat to bring the earrings to their melting point. The next step is to calculate how much heat is needed to melt the earrings. For this, use the heat of fusion (the latent heat) of gold:
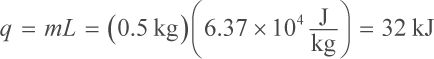
So overall, it requires approximately 60 + 32 kJ = 92 kJ of heat to melt the gold earrings. Notice that we can heavily approximate the numbers used in our calculations because the answer choices are so spread out. The closest answer is choice (D).
