MCAT Physics Question 130: Answer and Explanation
Home > MCAT Test > MCAT physics practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT physics practice tests.
Question: 130
10. Consider the following fission reaction.
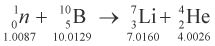
The masses of the species involved are given in atomic mass units below each species, and 1 amu can create 932 MeV of energy. What is the energy liberated due to transformation of mass into energy during this reaction?
- A. 0.003 MeV
- B. 1.4 MeV
- C. 2.8 MeV
- D. 5.6 MeV
Correct Answer: C
Explanation:
This problem presents a reaction and asks for the energy liberated due to transformation of mass into energy. To convert mass into energy, we are told that 1 amu can be converted into 932 MeV of energy. All we need to do now is calculate how much mass, in amu, is converted in the reaction. Because we are given the atomic mass for each of the elements in the reaction, this is simply a matter of balancing the equation:

This is the amount of mass that has been converted into energy. To obtain energy from mass, we have to multiply by the conversion factor (1 amu = 932 MeV):
E = 0.003 × 932 ≈ 0.003 × 900 = 2.7 MeV
