MCAT Physics Question 124: Answer and Explanation
Home > MCAT Test > MCAT physics practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT physics practice tests.
Question: 124
4. When a hydrogen atom electron falls to the ground state from the n = 2 state, 10.2 eV of energy is emitted. What is the wavelength of this radiation? (Note: 1 eV = 1.60 × 10-19 J, and h = 6.626 × 10-34 J·s.)
- A. 5.76 × 10-9 m
- B. 1.22 × 10-7 m
- C. 3.45 × 10-7 m
- D. 2.5 × 1015 m
Correct Answer: B
Explanation:
To solve this question correctly, one must be careful with the units. First, convert 10.2 eV to joules:
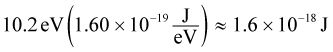
Next, to determine the wavelength of the radiation, first find the frequency:
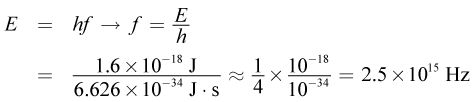
Lastly, from the wave equation c = f λ, we can calculate the wavelength of the radiation: