MCAT Organic Chemistry Question 39: Answer and Explanation
Home > MCAT Test > MCAT organic chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT organic chemistry practice tests.
Question: 39
9. How many σ bonds and π bonds are present in the following compound?
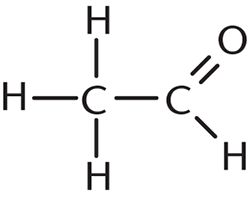
- A. Six σ bonds and one π bond
- B. Six σ bonds and two π bonds
- C. Seven σ bonds and one π bond
- D. Seven σ bonds and two π bonds
Correct Answer: A
Explanation:
Each single bond has one σ bond, and each double bond has one σ and one π bond. In this question, there are five single bonds (five σ bonds) and one double bond (one σ bond and one π bond), which gives a total of six σ bonds and one π bond. Thus, the correct answer is choice (A).
