MCAT Organic Chemistry Question 132: Answer and Explanation
Home > MCAT Test > MCAT organic chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT organic chemistry practice tests.
Question: 132
12. A positive charge on the molecule shown would have greater stability than a positive charge on a straight-chain alkane version of the same molecule. What property most explains this effect?
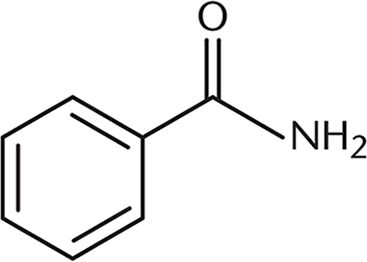
- A. Steric hindrance
- B. Nitrogen electronegativity
- C. Induction
- D. Conjugation
Correct Answer: D
Explanation:
This molecule is more stable with a positive charge than a straight-chain alkane due to the conjugation of the benzene ring. This permits delocalization of the charge through resonance. Although induction, choice (C), does have an effect on the stabilization of the molecule, this effect is much less significant than the impact of having a conjugated system. The electronegativity of nitrogen, choice (B), which primarily affects induction, is also not a vital component of the stabilization by this molecule of a positive charge because oxygen is more electronegative. Steric hindrance, choice (A), would affect the reactivity of a molecule, but not its ability to stabilize charge.
