MCAT Organic Chemistry Question 123: Answer and Explanation
Home > MCAT Test > MCAT organic chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT organic chemistry practice tests.
Question: 123
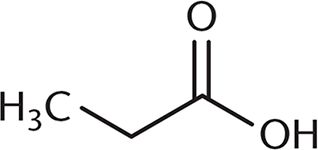
3. Which of the following undergoes a Fischer esterification most rapidly?
- A.
- B.
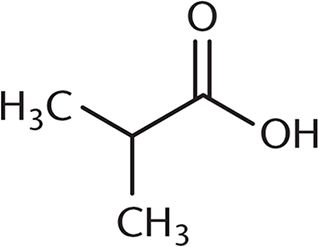
- C.
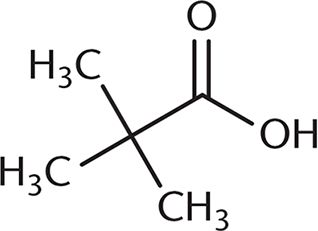
- D.
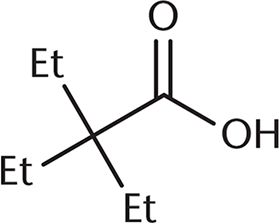
Correct Answer: A
Explanation:
A Fischer esterification involves reacting a carboxylic acid and an alcohol with an acid catalyst. Under these conditions, the carbonyl carbon is open to attack by the nucleophilic alcohol. The rate of this reaction depends on the amount of steric hindrance around the carbonyl carbon because there must be room for the alcohol to approach the carboxylic acid substrate. Choices (B), (C), and (D) all have more crowding around the carbonyl carbon, which will decrease reactivity. The additional alkyl groups in these other choices also donate electron density to the carbonyl carbon, making it slightly less electrophilic.
