MCAT Math Question 10: Answer and Explanation
Home > MCAT Test > MCAT math practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT math practice tests.
Question: 10
10. What is the approximate pH of a solution with a pKa of 3.6, [HA] = 100 mM, and [A-] = 0.1 M? (Note: 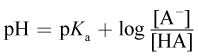 )
)
- A. 1.6
- B. 3.6
- C. 5.6
- D. 7.6
Correct Answer: B
Explanation:
This question involves both a unit conversion between millimolar values and molar values, and calculation of a logarithm. The relationship between pH and pKa is described by the Henderson–Hasselbalch equation given in the question stem. 100 mM = 0.1 M, so

