MCAT General Chemistry Question 99: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 99
9. Suppose 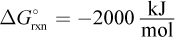 for a chemical reaction. At 300 K, what is the change in Gibbs free energy in
for a chemical reaction. At 300 K, what is the change in Gibbs free energy in 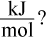
- A. ΔG = –2000 + (300 K)(8.314)(ln Q)
- B. ΔG = –2000 – (300 K)(8.314)(ln Q)
- C. ΔG = –2000 + (300 K)(8.314)(log Q)
- D. ΔG = –2000 – (300 K)(8.314)(log Q)
Correct Answer: A
Explanation:
This problem asks for the free energy of a reaction at nonstandard conditions, which can be determined with the equation ΔG = ΔG° + RT-ln Q.
