MCAT General Chemistry Question 98: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 98
8. Consider the chemical reaction in the vessel depicted in the following diagram.
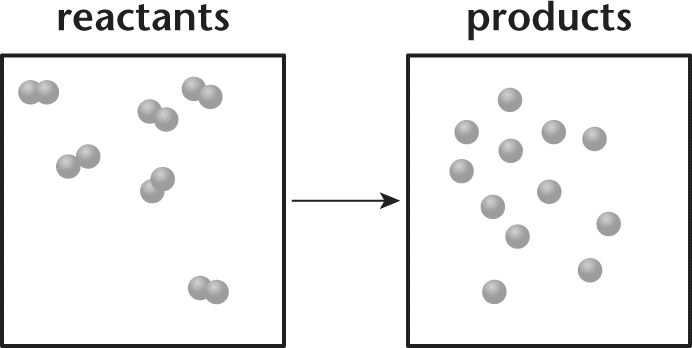
- A. The reaction is spontaneous.
- B. The reaction is nonspontaneous.
- C. The reaction is at equilibrium.
- D. There is not enough information to determine if the reaction is spontaneous.
Correct Answer: D
Explanation:
There is not enough information available to determine the free energy of this reaction. While the entropy is clearly increasing (there are more particles in the system), it is unclear what the enthalpy change is. Because bonds are breaking, the reaction must be endothermic, meaning that both ΔS and ΔH are positive. In this case, it is a temperature-dependent process, and—without a temperature given—we cannot determine the sign on ΔG.
