MCAT General Chemistry Question 95: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 95
5. Methanol reacts with acetic acid to form methyl acetate and water.
| Type of Bond | Bond Disassociation Energy 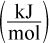 |
| C – C | 348 |
| C – H | 415 |
| C = O | 805 |
| O – H | 463 |
| C – O | 360 |
Based on the values in the table above, what is the heat of formation of methyl acetate in 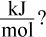
- A. 0
- B. 464
- C. 824
- D. 1288
Correct Answer: A
Explanation:
At first glance, this might seem like a math-heavy problem, but it really doesn't require any calculations at all. We just have to keep track of which bonds are broken and which bonds are formed. Remember, breaking bonds requires energy, while forming bonds releases energy. Two bonds are broken: a C-O bond between the carbon and oxygen of methanol, and an O-H bond between the hydroxyl oxygen and hydrogen of the acetic acid. Two bonds are also formed: a C-O bond between the esterifying group and the oxygen of the methyl acetate, and an O-H bond between the hydroxyl group and a hydrogen to form water. Given that the same two bonds are broken and formed in this reaction, the energy change must be