MCAT General Chemistry Question 91: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 91
1. Consider the cooling of an ideal gas in a closed system. This process is illustrated in the pressure–volume graph shown in the following figure.
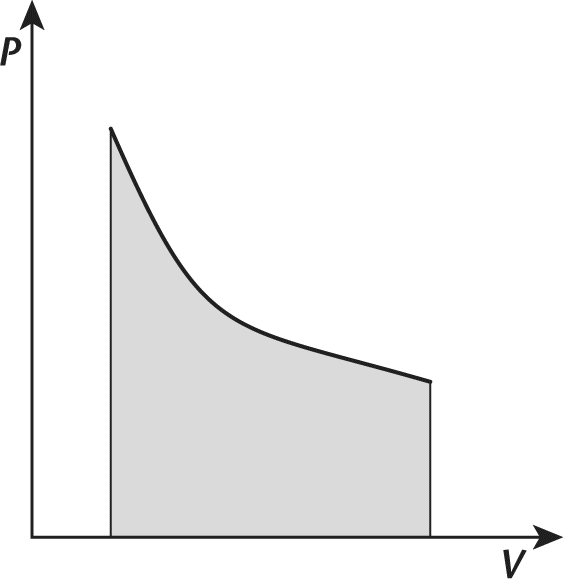
Based on this information, the process may be:
- A. adiabatic.
- B. isobaric.
- C. isothermal.
- D. isochoric.
Correct Answer: A
Explanation:
This process may be adiabatic. Given that the gas was cooled, it did not maintain constant temperature, eliminating choice (C). Isobaric and isovolumetric processes appear as horizontal and vertical lines in pressure-volume graphs, respectively, eliminating choices (B) and (D). Adiabatic processes appear hyperbolic on pressure-volume graphs, as illustrated here.
