MCAT General Chemistry Question 79: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 79
4. What is the proper equilibrium expression for the reaction below?
2 NO2 (g) + 4 H2 (g) ? N2 (g) + 4 H2O (g)
- A.
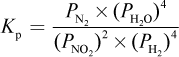
- B.
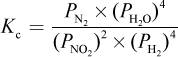
- C.
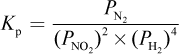
- D.
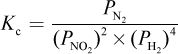
Correct Answer: A
Explanation:
Recall that equilibrium constants are either based on concentrations (Kc) or partial pressures (Kp). In this case, because all species are in the gas phase, we are using Kp—eliminating choices (B) and (D). When water is in the liquid phase, it does not appear in equilibrium expressions, as in choice (C). Here, however, water is in the gaseous phase and thus should appear in the equilibrium expression.
