MCAT General Chemistry Question 75: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 75
15. The potential energy diagram shown represents four different reactions.
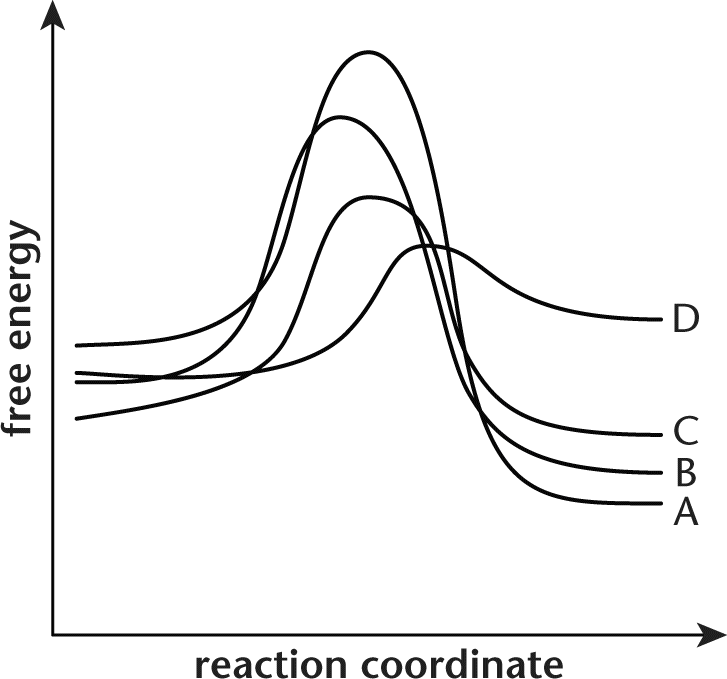
Assuming identical conditions, which of the reactions displayed on the energy diagram proceeds the fastest?
- A. A
- B. B
- C. C
- D. D
Correct Answer: D
Explanation:
The faster a reaction can reach its activation energy, the faster it will proceed to completion. Because this question states that all conditions are equal, the reaction with the lowest activation energy will have the fastest rate. In the diagram, choice (D) has the lowest activation energy.
