MCAT General Chemistry Question 72: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 72
12. For question below, consider the following energy diagram shown below:
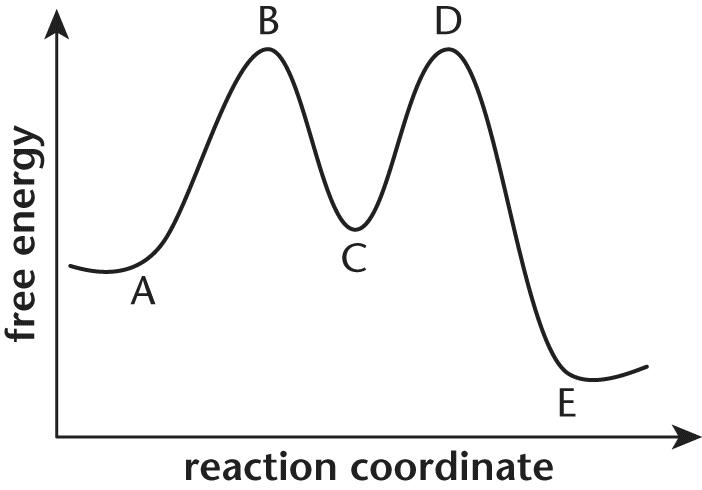
Which process has the highest activation energy?
- A. The first step of the forward reaction
- B. The first step of the reverse reaction
- C. The second step of the forward reaction
- D. The second step of the reverse reaction
Correct Answer: B
Explanation:
The activation energy of a reaction is represented by the distance on the y-axis from the energy of the reactants to the peak energy prior to formation of products. The activation energy of the first step of the forward reaction, for example, is equal to the distance along the y-axis from point A to point B. The largest energy increase on this graph occurs during the progress from point E to point D, which represents the first step of the reverse reaction.
