MCAT General Chemistry Question 71: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 71
11. For question below, consider the following energy diagram shown below:
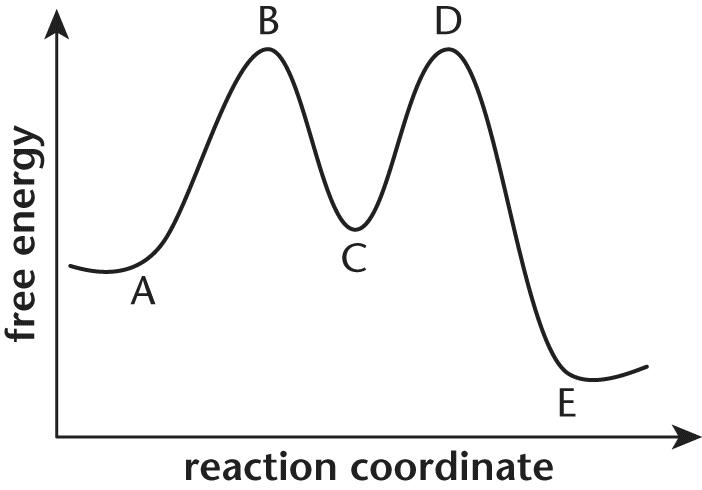
The overall reaction depicted by this energy diagramis:
- A. endergonic, because point B is higher than point A.
- B. endergonic, because point C is higher than point A.
- C. exergonic, because point D is higher than point E.
- D. exergonic, because point A is higher than point E.
Correct Answer: D
Explanation:
A system is exergonic if energy is released by the reaction. For exergonic reactions, the net energy change is negative, and the free energy of the final products is lower than the free energy of the initial reactants. Point E, which represents the of the final products, is lower on the energy diagram than point A, which represents the energy of initial reactants. Thus, energy must have been given off, and the reaction is exergonic.
