MCAT General Chemistry Question 68: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 68
8. The following data shown in the table were collected for the combustion of the theoretical compound XH4:
XH4 + 2 O2 → XO2 + 2 H2O
| Trial | [XH4]initial (M) | [O2]initial (M) | 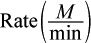 |
| 1 | 0.6 | 0.6 | 12.4 |
| 2 | 0.6 | 2.4 | 49.9 |
| 3 | 1.2 | 2.4 | 198.3 |
What is the rate law for the reaction described here?
- A. rate = k[XH4][O2]
- B. rate = k[XH4][O2]2
- C. rate = k[XH4]2[O2]
- D. rate = k[XH4]2[O2]2
Correct Answer: C
Explanation:
In the first two trials, the concentration of XH4 is held constant, while the concentration of O2 is multiplied by 4. Because the rate of the reaction is also increased by a factor of approximately 4, oxygen must be a first-order reactant. In the last two trials, the concentration of O2 is held constant while the concentration of XH4 is doubled. Because the rate of the reaction is increased by a factor of approximately 4 again, XH4 must be a second-order reactant. Based on this, we can conclude that the experimental rate law is k[XH4]2[O2].
