MCAT General Chemistry Question 62: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 62
2. In a certain equilibrium process, the activation energy of the forward reaction is greater than the activation energy of the reverse reaction. This reaction is:
- A. endothermic.
- B. exothermic.
- C. spontaneous.
- D. nonspontaneous.
Correct Answer: D
Explanation:
Before you try to answer this question, you should draw a free energy diagram for the system.
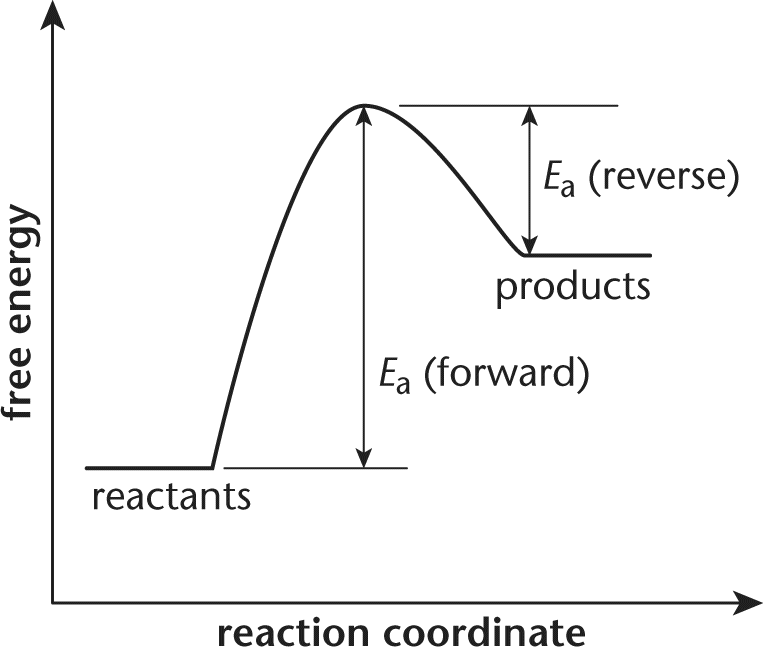
If the activation energy of the forward reaction is greater than the activation energy of the reverse reaction, then the products must have a higher free energy than the reactants. The overall energy of the system is higher at the end than it was in the beginning. The net free energy change is positive, indicating an endergonic (nonspontaneous) reaction. The terms endothermic, choice (A), and exothermic, choice (B), are associated with enthalpy. While free energy does depend on enthalpy, it also depends on entropy; there is not enough information in the question stem to reliably determine the sign of the enthalpy change of the reaction.
