MCAT General Chemistry Question 61: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 61
1. In a third-order reaction involving two reactants and two products, doubling the concentration of the first reactant causes the rate to increase by a factor of 2. What will happen to the rate of this reaction if the concentration of the second reactant is cut in half?
- A. It will increase by a factor of 2.
- B. It will increase by a factor of 4.
- C. It will decrease by a factor of 2.
- D. It will decrease by a factor of 4.
Correct Answer: D
Explanation:
Based on the information given in the question, the rate is first-order with respect to the concentration of the first reactant; when the concentration of that reactant doubles, the rate also doubles. Because the reaction is third-order, the sum of the exponents in the rate law must be equal to 3. Therefore, the reaction order with respect to the other reactant must be 3 - 1 = 2. If the concentration of this second reactant is multiplied by 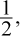 the rate will be multiplied by
the rate will be multiplied by