MCAT General Chemistry Question 60: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 60
15. What is the molecular formula of a compound with an empirical formula of B2H5 and a molar mass of 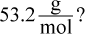
- A. B2H5
- B. B3H7
- C. B4H10
- D. B6H15
Correct Answer: C
Explanation:
The simplest approach is to determine the molar mass of the empirical formula. B2H5 has a molar mass of 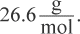 A molecular formula is simply a multiple of the empirical formula; doubling this quantity will result in the molar mass given in the question stem. Therefore, the compound must be B4H10.
A molecular formula is simply a multiple of the empirical formula; doubling this quantity will result in the molar mass given in the question stem. Therefore, the compound must be B4H10.
