MCAT General Chemistry Question 57: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 57
12. In the process of photosynthesis, carbon dioxide and water combine with energy to form glucose and oxygen, according to the following equation:
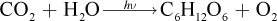
What is the theoretical yield of glucose if 30 grams of water are reacted with excess carbon dioxide and energy, according to the equation above?
- A. 30.0 g
- B. 50.0 g
- C. 300.1 g
- D. 1801 g
Correct Answer: A
Explanation:
The equation given is unbalanced. To start, we must balance the equation:
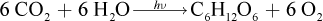
The theoretical yield is the amount of product synthesized if the limiting reagent is completely used up. This question therefore asks how much glucose is produced if the limiting reagent is 30 grams of water. We can use the three-fraction method discussed in this chapter to solve for the mass of glucose produced:
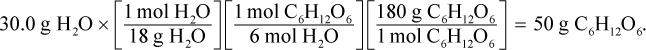
Thus, 50 grams of glucose are produced.
