MCAT General Chemistry Question 53: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 53
8. Using a given mass of KClO3, how would one calculate the mass of oxygen produced in the following reaction, assuming it goes to completion?
2 KClO3 → 2 KCl + 3 O2
- A.
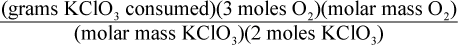
- B.
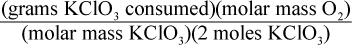
- C.
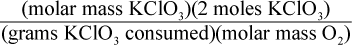
- D.
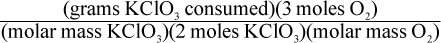
Correct Answer: A
Explanation:
This is a question best answered by dimensional analysis. Keeping in mind that molar mass is measured in grams of a substance per moles of that substance, only choice (A) comes out with the units of grams of oxygen. Choice (B) has the units of grams per mole of oxygen, not grams of oxygen. Choice (C) has the units of moles per gram of oxygen. Choice (D) has the units of mol2 per gram of oxygen.
