MCAT General Chemistry Question 50: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 50
5. In which of the following compounds is the percent composition of carbon by mass closest to 62 percent?
- A. Acetone
- B. Ethanol
- C. C3H8
- D. Methanol
Correct Answer: A
Explanation:
The percent composition by mass of any given element within a molecule is equal to the mass of that element in the molecule divided by the molar mass of the compound, times 100%. In this case, acetone, C3H6O, has
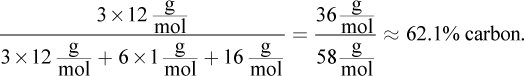
Choice (B), ethanol, is
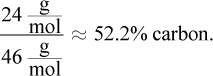
Choice (C), propane, is
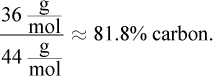
Finally, choice (D), methanol, is