MCAT General Chemistry Question 49: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 49
4. Which of the following molecules CANNOT be expressed by the empirical formula CH?
- A. Benzene
- B. Ethyne
- C.
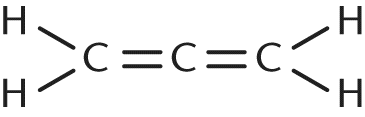
- D.
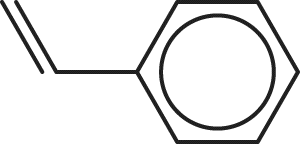
Correct Answer: C
Explanation:
The definition of an empirical formula is a formula that represents a molecule with the simplest ratio, in whole numbers, of the elements comprising the compound. In this case, given the empirical formula CH, any molecule with carbon and hydrogen atoms in a 1:1 ratio would be accurately represented by this empirical formula. Choice (C) is has three carbon atoms and four hydrogen atoms. Both its molecular and empirical formulas would be C3H4 because this formula represents the smallest whole-number ratio of its constituent elements.
