MCAT General Chemistry Question 48: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 48
3. Which of the following is the gram equivalent weight of H2SO4 with respect to protons?
- A. 49.1 g
- B. 98.1 g
- C. 147.1 g
- D. 196.2 g
Correct Answer: A
Explanation:
First, it is helpful to know the molar mass of one mole of H2SO4, which is found by adding the atomic weights of the atoms that constitute the molecule:
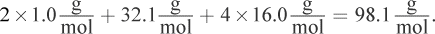
Gram equivalent weight is the weight (in grams) that would release one mole of protons.Because sulfuric acid has two hydrogens per molecule, the gram equivalent weight is 98.1 g divided by 2, or 49.1 g.
