MCAT General Chemistry Question 43: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 43
13. Which of the following is the best name for the new bond formed in the reaction shown?
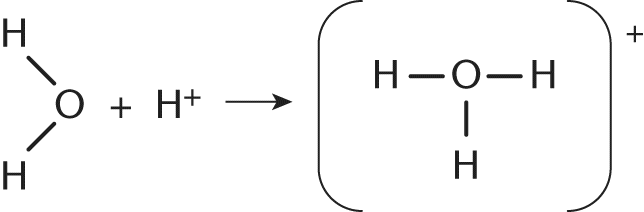
- A. Nonpolar covalent bond
- B. Ionic bond
- C. Coordinate covalent bond
- D. Hydrogen bond
Correct Answer: C
Explanation:
The reaction in this question shows a water molecule, which has two lone pairs of electrons on the central oxygen, combining with a free hydrogen cation. The resulting molecule, H3O+ has formed a new bond between H+ and H2O. This bond is created via the sharing of one of oxygen's lone pairs with the free H+ ion. This represents the donation of a shared pair of electrons from a Lewis base (H2O) to a Lewis acid (H+, electron acceptor). This type of bond is called a coordinate covalent bond.
