MCAT General Chemistry Question 42: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 42
12. In the structure shown, which atom(s) have the most positive charge?
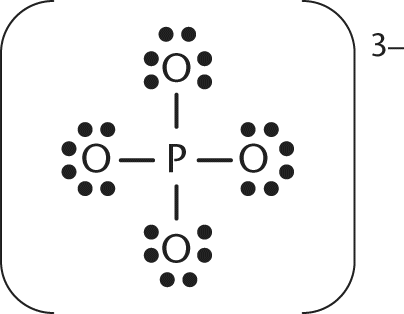
- A. The phosphorus atom has the most positive charge.
- B. All atoms share the charge equally.
- C. The four oxygens share the highest charge.
- D. The oxygen at the peak of the trigonal pyramidal geometry has the most positive charge.
Correct Answer: A
Explanation:
In this Lewis diagram, the phosphate molecule has an overall formal charge of -3. The four oxygens would each be assigned a formal charge of -1. Given the overall charge of -3 and the -1 charge on each oxygen, the phosphorus must have a formal charge of +1.
