MCAT General Chemistry Question 33: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 33
3. Which of the following structure(s) contribute most to NO2's resonance hybrid?
I. 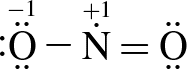
II. 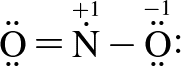
III. 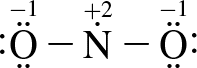
- A. I only
- B. III only
- C. I and II only
- D. I, II, and III
Correct Answer: C
Explanation:
The two greatest contributors are structures I and II. Resonance structures are representations of how charges are shared across a molecule. In reality, the charge distribution is a weighted average of contributing resonance structures. The most stable resonance structures are those that minimize charge on the atoms in the molecule; the more stable the structure, the more it will contribute to the overall charge distribution in the molecule. Structures I and II minimize formal charges, so will be the largest contributors to the resonance hybrid.
