MCAT General Chemistry Question 32: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 32
2. Which of the following molecules contains the oxygen atom with the most negative formal charge?
- A. H2O
- B. CO32–
- C. O3
- D. CH2O
Correct Answer: B
Explanation:
To answer this question, one must understand the contribution of resonance structures to average formal charge. In , there are three possible resonance structures. Each of the three oxygen atoms carries a formal charge of -1 in two out of the three structures. This averages to approximately 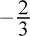 charge on each oxygen atom, which is more negative than in the other answer choices. Both water and formaldehyde, choices (A) and (D), have no formal charge on the oxygen. Ozone, choice (C), has a
charge on each oxygen atom, which is more negative than in the other answer choices. Both water and formaldehyde, choices (A) and (D), have no formal charge on the oxygen. Ozone, choice (C), has a 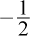 on two of the three oxygens and a +1 charge on the central oxygen.
on two of the three oxygens and a +1 charge on the central oxygen.
