MCAT General Chemistry Question 298: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 298
5. What is the expected potential of the cell at the start of the experiment?
- A. +0.118 V
- B. +0.059 V
- C. -0.118 V
- D. -0.059 V
Correct Answer: B
Explanation:
B A concentration cell is spontaneous, eliminating the negative values of potential in choices C and D. The net "reaction" of the concentration cell in the passage is:
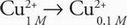
Therefore, the reaction quotient is:
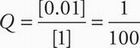
Copper is reacting between the Cu and Cu(II) state, so n = 2. Substitute these quantities into the Nernst equation to solve for potential: