MCAT General Chemistry Question 286: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 286
5. The shape of the carbonate ion is:
- A. trigonal planar and the carbon atom is sp3 hybridized.
- B. trigonal planar and the carbon atom is sp2 hybridized.
- C. tetrahedral and the carbon atom is sp2 hybridized.
- D. tetrahedral and the carbon atom is sp3 hybridized.
Correct Answer: B
Explanation:
B The carbonate ion is trigonal planar and the carbon atom is sp2 hybridized:
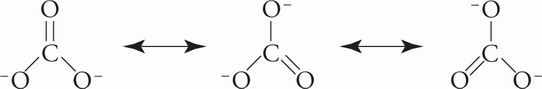
With three total substituents, the carbon atom cannot be sp3 hybridized, eliminating choices A and D. Tetrahedral shapes require four substituents, eliminating choice C.
