MCAT General Chemistry Question 284: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 284
3. What is the approximate concentration of CO3-2 when enough CaCO3(s) is dissolved in water to form a saturated solution?
- A. 2.5 × 10-9 M
- B. 5.0 × 10-9 M
- C. 6.0 × 10-5 M
- D. 1.0 × 10-4 M
Correct Answer: C
Explanation:
C Calcium carbonate dissolves in water as follows:
CaCO3(s) 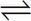 Ca2+ + CO32-
Ca2+ + CO32-
The Ksp defines the maximum solubility of a saturated solution. Solve the solubility expression for the maximum theoretical concentration of CO32-:
Ksp = [Ca2+][CO2-3]
3.4 × 10-9 = [x][x]
x2 = 34 × 10-10
x ≈ 6 × 10-5M
