MCAT General Chemistry Question 276: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 276
2. If Step 1 were simply a fast reaction and not a fast equilibrium, what would be the expected rate law for the decomposition of nitramide in water?
- A. Rate = k[O2NNH2]
- B. Rate = k[O2NNH-]
- C. Rate = k[H+][OH-]
- D. Rate =
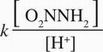
Correct Answer: B
Explanation:
B If the first step was simply a fast step, then we would be able to make the normal assumptions about elementary steps and rate laws. More specifically, the rate law could be determined by the stoichiometry of the slow Step 2, which would yield the rate law in choice B.
