MCAT General Chemistry Question 274: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 274
6. If a 20 L sample of gas at STP were cooled to mesosphere temperatures at a constant volume, what would the new pressure be?
- A. 64 kPa
- B. 84 kPa
- C. 96 kPa
- D. 128 kPa
Correct Answer: A
Explanation:
A STP is 1 atm ≈ 100 kPa and 0°C = 273 K. Solve for the new pressure at the mesosphere temperature given in the passage (-100°C = 173 K) using the combined gas law (since volume is constant, V drops out of the equation):
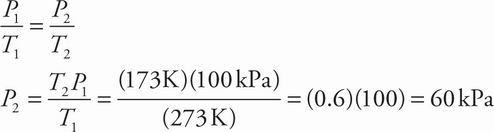
Note that pressure must decrease at decreased temperature, eliminating choice D.
