MCAT General Chemistry Question 27: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 27
12. Of the four atoms depicted here, which has the highest electron affinity?
- A.
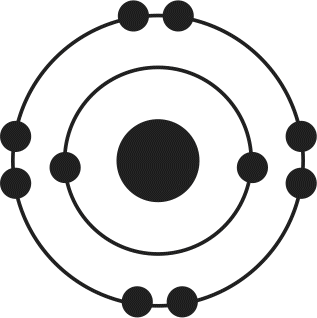
- B.
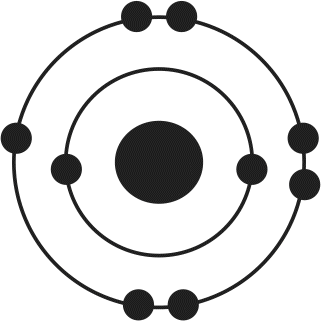
- C.
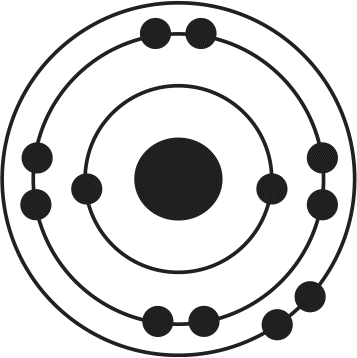
- D.
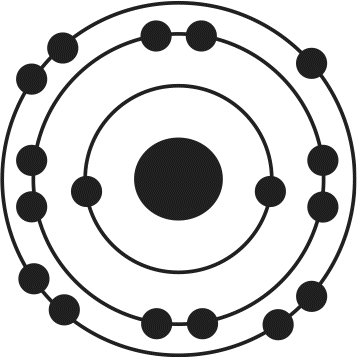
Correct Answer: B
Explanation:
Electron affinity is related to several factors, including atomic size and filling of the valence shell. As atomic radius increases, the distance between the nucleus and the outermost electrons increases, thereby decreasing the attractive forces between protons and electrons. As a result, increased atomic radius will lead to lower electron affinity. Because atoms are in a low-energy state when their outermost valence electron shell is filled, atoms needing only one or two electrons to complete this shell will have high electron affinities. In this example, choices (B) and (D) need only one more electron to have a noble gas-like electron configuration; because choice (B) is smaller, it will have the highest electron affinity.
