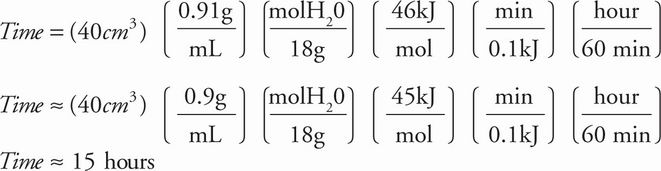MCAT General Chemistry Question 268: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 268
5. The heat of sublimation of water is 46 kJ/mol. If heat is transferred to the sample by the environment at a rate of 0.1 kJ/min, approximately how long will it take to lyophilize 40 cm3 of frozen water (density = 0.91 g/mL) ?
- A. 7.7 hours
- B. 15.3 hours
- C. 77.0 hours
- D. 153.0 hours
Correct Answer: B
Explanation:
B 40 cm3 of ice is 36 g or 2 moles of water. The heat required to sublimate this sample is 46 kJ/mol(2 mol) = 92 kJ. If heat is transferred at 0.1 kJ/min, then 920 minutes are required. Dividing 920 min by 60 min/hour gives just over 15 hours. Overall: