MCAT General Chemistry Question 257: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 257
6. For a diatomic molecule, the reduced mass is given by u = (m1 × m2) / (m1 + m2) where m1 and m2 are the atomic weights of the two bonded atoms. What will be the ratio of the ground state vibration energies of D2 to H2 assuming the force constant k is the same for both?
- A. 0.5
- B. 0.7
- C. 1.4
- D. 2.0
Correct Answer: B
Explanation:
B Deuterium (D) has one proton, one neutron, and an atomic mass of 2. For D2, u = (2 × 2) / (2 + 2) = 1. For H2, u = 1 × 1 / (1 + 1) = 1/2. Since v, h, and k are all constant in this comparison, the vibration energy equation in the passage reduces as follows:
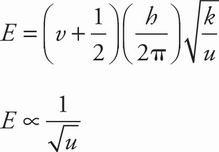
The ratio of this energy for D2 to H2 is:
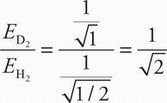
The quantity must be greater than 0.5 but less than 1, so only choice B is possible.
