MCAT General Chemistry Question 254: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 254
3. Assuming their reduced masses are the same, which molecule will have the highest energy of vibration in the v = 0 state?
- A. N2
- B. O2
- C. F2
- D. Cannot be determined from the information given.
Correct Answer: A
Explanation:
A If v = 0 and h and u are constant, the equation for vibration energy reduces as follows:
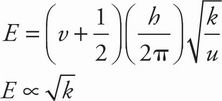
The energy of the ground state is proportional to the square root of the force constant k. The passage states that k is larger for stronger bonds, and the bond in N2 is strongest since it has the highest bond energy (see Table 1). N2 must have the highest vibrational energy in the ground state.
