MCAT General Chemistry Question 244: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 244
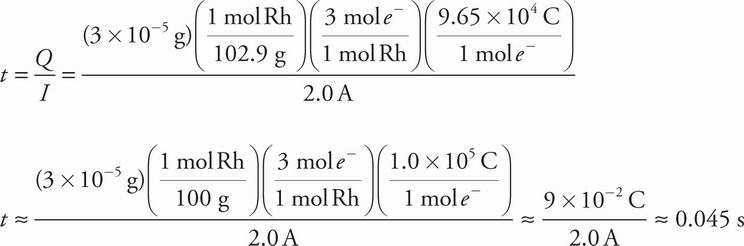
6. To give "white gold" a white appearance, it is plated with rhodium by immersion in a rhodium sulfate solution (Rh2(SO4)3(aq)). Provided with a current of 2.0 A, how long must a 3.0 g white gold broach be immersed to plate 3.0 × 10-5 g of rhodium? (Faraday's constant = 96,500 C/mol e-)
- A. 0.0009 s
- B. 0.0098 s
- C. 0.042 s
- D. 0.56 s
Correct Answer: C
Explanation:
C Since current (I) = charge(Q) / time(t) we can set up and solve the following equation keeping in mind that the rhodium reduction in question is Rh3+ + 3e- → Rh(s).