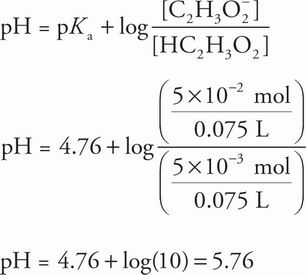MCAT General Chemistry Question 238: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 238
7. A 25.0 mL solution of 0.2 M acetic acid (pKa = 4.76) is mixed with 50 mL of 1.0 M sodium acetate (pKb = 9.24). What is the final pH?
- A. 4.8
- B. 5.8
- C. 9.2
- D. 10.2
Correct Answer: B
Explanation:
B The sodium acetate solution will be completely ionized:
NaC2 H3O2 → Na + C2H3 O2-
However, acetic acid will have negligible dissociation in solution:
HC2H3O2 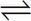 H+ + C2H3O2- (Ka ≈ 1 × 10-5)
H+ + C2H3O2- (Ka ≈ 1 × 10-5)
Therefore, for the combined solution, it is reasonable to assume that all of the HC2H3O2 is contributed from the acid solution, and all of the C2H3O2- is contributed from the salt solution:
(0.2 M HC2H3O2)(0.025 L) = 5 × 10-3 mol HC2H3O2
(1 M NaC2H3O2)(0.05 L) = 5 × 10-2 mol C2H3O2-
The new volume of 0.075 L cancels out when solving for the pH using the Henderson-Hasselbalch equation: