MCAT General Chemistry Question 236: Answer and Explanation
Home > MCAT Test > MCAT general chemistry practice tests
Test Information
- Use your browser's back button to return to your test results.
- Do more MCAT general chemistry practice tests.
Question: 236
5. The amino and carboxyl terminals of alanine lose protons according to the following equilibrium:
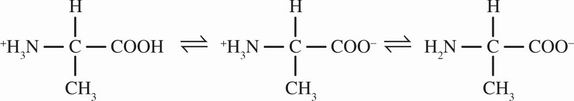
Which of the following indicators would be best used to determine the second equivalence point when alanine is titrated with sodium hydroxide?
- A. Methyl violet (pKb = 13.0)
- B. Methyl yellow (pKb = 10.5)
- C. Thymol blue (pKb = 12.0)
- D. Phenolphthalein (pKb = 4.9)
Correct Answer: D
Explanation:
D Alanine is a neutral amino acid with an isoelectric point close to 7. Therefore, the second equivalence point represents when all the ammonium residue of the zwitterion (the middle structure shown in the question) is deprotonated. This must occur at a basic pH. An appropriate indicator will change color if its pKa is ±1 of the pH at this equivalence point. Therefore, the desired indicator should have a pKa > 7, or pKb < 7, making choice D the best answer. Another approach to this question is to recognize that no numerical data are provided and choice D is the only indicator for a basic region. There would be no other reasonable way to choose between choices A, B, and C.
